Research
During the last ten years, our main efforts have been directed to increase the therapeutic efficacy of nanomedicine and develop the new supramolecular cancer therapy. In the nanomedicine approach, we can increase the encapsulation stability of small drug molecules and minimize protein corona formation by supramolecularly polymer/protein modification onto mesoporous silica nanoparticle. However, molecular mechanism of the interactions occurring on the interfaces between nanomaterials and proteins still remains largely unknown and furthermore there is a challenge to regulate the nanoparticle–biological interactions that provide profound impacts on cancer therapy. As an expansion of the previous research, we plan to further investigate these interactions by varying nanoparticles including biodegradable silica nanoparticle, mesorporous metal-ogranic framework, and proteins. Eventually, based on the fundamental understanding of these interactions, we will investigate the method to become closer to clinical translation of nanomedicine. In the supramolecular therapy, we showed a new paradigm in cancer therapy, drug-free cancer therapy, which can overcome the multidrug resistance, limitation of current chemotherapy. However, it still has a limitation at the selectivity to cancer and pharmacokinetics. Thus, we plan to develop tumor microenvironment responsive supramolecular therapy to increase tumor selectivity and vary molecular design of peptides to enhance pharmacokinetics.
The research theme of the Ryu research group is the development of new disease therapy using supramolecular approach (Supramolecular Therapeutics). The Ryu group have developed the conventional-drug-free approach for a new anti-cancer/anti-ageing therapy, that intracellular (supramolecular) polymerization inside the mitochondria induced the dysfunction of mitochondria by disrupting the membrane, resulting in the selective apoptosis of cancer/senescent cells. In addition, the Ryu group have developed a facile synthetic method for highly stable, polymer/protein-modified hollow nanoparticles using a non-covalent approach to enhance in vivo efficacy and target ability to caner and senescent cells.
Intracellular Bioactive Supramolecular Assembly
The formation of most biological nanostructures is driven by self-assembly processes and the structured biomaterials have biochemical activities such as enzyme activity and protein signaling. The artificial assembly of synthetic building units inside a living cell and the interaction of these units with the cellular components have rarely been studied, but are emerging as an intriguing strategy to control cellular fate. In particular, self-assembly inside cellular organelles is challenging because of the practical difficulty in observing the complex intracellular environment, and thus has not yet been reported. Achievement of artificial self-assembly of small molecules inside such organelles could be an advanced strategy for an efficient external control over organelle function and manipulation of the cellular fate.
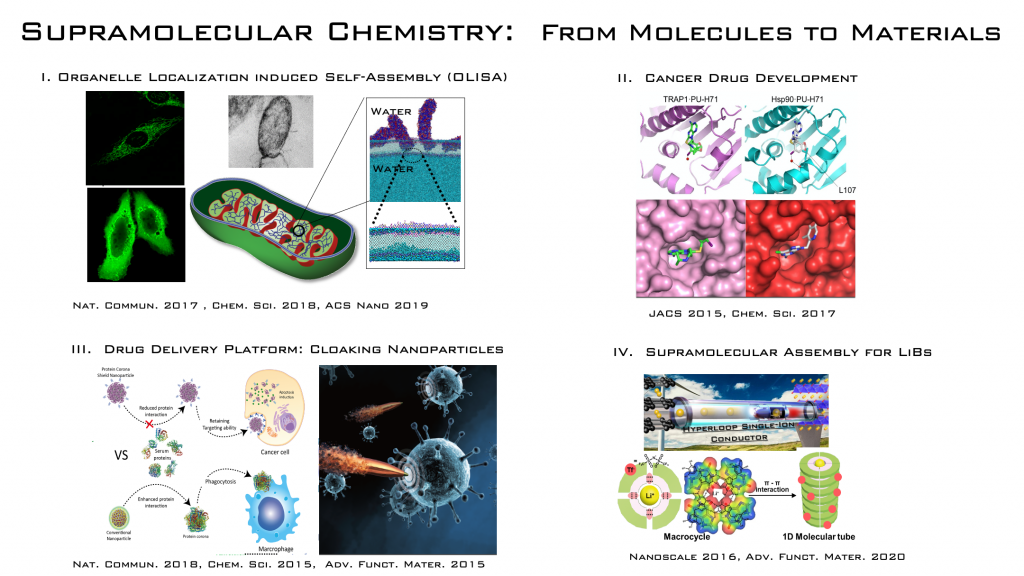
Organelle Localization Induced Self assembly of Peptide Amphiphile to Control the Cell fate
Self assembly is a well established phenomenon over decades, however self assembly of building units to form interesting nano structures inside living cells are something amazing and is exciting when they could control the cellular fate like proliferation, apoptosis and metabolism. Self assembly is an equilibrium process between the individual building units and their aggregated state, and the concentration of the molecules should be over the critical value to induce assembly. So it is critical to think about reducing the effective concentration for more practicability. An Organalle Localization Induced Self assembly (OLISA) is more promising and reliable. In here, the building units accumulate inside cellular organelle, thereby increasing the effective concentration (~500-1000) compared with feeding concertation. OLISA is a pioneering approach which open up huge possibility to control the cell fate as well as investigating cellular pathways and functions.
The artificial assembly of synthetic building units inside a living cell and the interaction of these units with the cellular components have rarely been studied, but are emerging as an intriguing strategy to control cellular fate. In particular, self-assembly inside cellular organelles is challenging because of the practical difficulty in observing the complex intracellular environment, and thus has been rarely reported. Achievement of artificial self-assembly of small molecules inside such organelles could be an advanced strategy for an efficient external control over organelle function and manipulation of the cellular fate. Considering the role of vicious mitochondrial fibril proteins such as amyloid beta (Aβ) in Alzheimer’s disease, we hypothesized that artificial induction of fibril formation inside the mitochondria could promote mitochondrial dysfunction and induce cell damage. Amphiphilic peptides with a mitochondrial targeting unit selectively accumulate in the mitochondria and self-assemble into an ordered structure because inside the confined organelle, the concentration of the peptides is significantly increased over their critical aggregation concentration. The fibrous structure inside the mitochondria then disrupts the mitochondrial membrane to cause leakage of the mitochondrial contents into the cytosol, resulting in severe damage to the cells. This was the first example showing spatiotemporal self-assembly of peptide amphiphiles to cause mitochondrial dysfunction.
Nanomedicine for Cancer Therapy
Noncovalent Polymer Gatekeeper in Mesoporous Nanparticles
Non-covalently binding drug molecules and then releasing them in response to an external trigger has been an important goal. Mesoporous silica nanoparticles with gatekeeper strategies could release the drug molecules in response to specific stimuli. However, it requires complex chemical modification of mesoporous silica nanoparticles, hence limiting their capability to encapsulate high amount of drug and versatility for ligand functionalization. We developed a novel polymer gatekeeper that can noncovalently block the pores of mesoporous silica nanoparticles and be simply modified with targeting ligands. Hydrophilic/hydrophobic drug molecules can be encapsulated at high doses, since the mesoporous silica nanoparticles are not chemically modified, thereby providing maximum pore volume. Moreover, noncovalently encapsulated drugs can be released in response to intracellular glutathione concentrations after cellular internalization by receptor-mediated uptake.
Protein Gatekeeper in Mesoporous Nanparticles
As a ‘Magic Bullet’ concept, cancer targeting nanomaterials have been extensively attempted by coating their surfaces with small molecules, peptides, proteins, and antibodies, but the tumor targeting and therapeutic efficacy still exhibits only a modest improvement. There is increasing evidence (Dawson et al. Nat Nanotechnol(2013), Stauber et al. Nat Nanotechnol(2013), and Wurm et al. Nat Nanotechnol(2016)) that the inefficient targeting can be associated with deleterious interactions of nanomaterials with proteins and consequent change (protein corona formation) on the surfaces when exposed to physiological environments. However, molecular mechanism of the interactions occurring on the interfaces between nanomaterials and proteins remains largely unknown and furthermore there is a challenge to regulate the nanoparticle–biological interactions that provides profound impacts on cancer therapy. We showed the development of supramolecularly pre-coated protein corona shield on nanoparticles and the investigation for the mechanism involved in regulating the nanoparticle– biological interactions. We developed mesoporous nanoparticles pre-coated with a recombinant fusion protein featuring HER2-binding affibody molecule and glutathione-S-transferase. Upon stabilized in preferred orientations on nanoparticle, the pre-coated proteins as a corona minimize interactions with serum proteins to prevent the clearance of these particles by macrophages, while ensuring their targeting function in vitro and in vivo. These findings provide a new targeting platform for the biomedical community.
Nanomachine for Cell Penetration
Biological nanomachines, including proteins and nucleic acids whose function is activated by conformational changes, are involved in every biological process, in which their dynamic and responsive behaviors are controlled by supramolecular recognition. The development of artificial nanomachines that mimic the biological functions for potential application as therapeutics is emerging; however, it is still limited to the lower hierarchical level of the molecular components. We develop a synthetic machinery nanostructure in which actuatable molecular components are integrated into a hierarchical nanomaterial in response to external stimuli to regulate biological functions. Two nanometers core-sized gold nanoparticles are covered with ligand layers as actuatable components, whose folding/unfolding motional response to the cellular environment enables the direct penetration of the nanoparticles across the cellular membrane to disrupt intracellular organelles. Furthermore, the pH-responsive conformational movements of the molecular components can induce the apoptosis of cancer cells. This strategy based on the mechanical motion of molecular components on a hierarchical nanocluster would be useful to design biomimetic nanotoxins.
Mitochondria Targeting Drug
Mitochondrial TRAP1 inhibitor
The crystal structures of human TRAP1 complexed with Hsp90 inhibitors conjugated to a mitochondria-targeting moiety was developed, and we investigated the rational development of a mitochondria-targeted Hsp90 inhibitor to specifically inactivate TRAP1. We showed crystal structures of both the open and closed TRAP1 conformations, and can, therefore, suggest molecular mechanisms of conformational change during the TRAP1 ATPase cycle, which will also aid in understanding the general mechanisms of Hsp90 chaperone function. In regard to development of mitochondrial Hsp90 inhibitors as cancer drugs, we clearly demonstrated that TRAP1 predominates over Hsp90 in cancer cell mitochondria and showed limitations of current Hsp90 inhibitors for inactivating the mitochondrial pool of TRAP1, primarily due to inefficient accumulation in mitochondria. Instead of designing specific inhibitors to the TRAP1 ATP pocket, we generated mitochondrial TRAP1-selective inhibitors by modifying current Hsp90 inhibitors to become “mitochondria-specific”. The concept of “organelle-specific” can be broadly applied to increase drug efficacy and minimize side effects for many drugs targeting proteins in mitochondria.
Mitochondrial Targeted Photodynamic Therapy
Photodynamic therapy (PDT) has widely accepted as a non-invasive tool for cancer treatment. However, there are few limiting factors that retard the therapeutic efficacy of the techniques. Targeting mitochondria has proven to be an effective strategy in many treatment modalities. Near IR absorbing dye, particularly IR-780 was widely used in PDT because of their high absorption in the far red/NIR region and mitochondria targeting ability. However, poor photostabilty, water solubility, and low mitochondrial co-localization limit the efficacy. We introduced a new indocyanine derivative (IR-Pyr), which was chemically modified with two pyridinium ions, to make highly water soluble, exhibit higher mitochondrial localization and better photostability than IR-780. In order to make it more specific to cancer-mitochondria we constructed a supramolecular aggregate using electrostatic interactions between the positively charged IR-Pyr and negatively charged hyaluronic acid (HA). The cancermitochondria targeting strategy assures the high PDT efficacy that proved by in vitro and in vivo experiments.
The low selectivity of sensitizers for cancer cells has still been an issue of the unwanted side effect into healthy tissue and overexpression of antiapoptotic agents during PDT is a major hurdle to attaining high therapeutic efficacy. We designed and synthesized a multifunctional conjugate IR-PU that addresses these issues. IR-PU contains mitochondria targeting group (pyridinium), heat shock protein (HSP) inhibitor (PU-H71 moiety), and the photosensitizer (indocyanine derivative). IR-PU binds Hsp90 family proteins such as Hsp90 and TRAP1 with 100-times greater affinity in cancer cells than it does the inactive forms of these proteins in normal cells, leading to selective accumulation in cancer cells and concomitant inhibition of their molecular chaperon activity. Furthermore, mitochondria-targeted PDT can rapidly damage the biological functions of the organelles under photoactivation, leading to the death of tumor cells. The approach will be useful in the production of a new generation of active molecules with improved therapeutic efficacy.
Supramolecular Assembly for Materials
Binder for Silicon Anodes in Lithium-Ion Batteris
Lithium-ion batteries (LIBs) have emerged over the past decade as one of the most promising energy storage and delivery devices because of their high energy densities and high energy efficiencies. Among anode materials in development for LIBs, silicon (Si) has great potential because of its high theoretical charge capacity, 4200 mAh g–1 which is almost ten times greater than those of the conventional graphite anode (372 mAh g–1). However, the practical application of Si in LIBs is challenging due to its mechanical fractures and the pulverization of the Si anode resulting from the large volume expansion that the material experiences during lithiation. Structuring of Si particles and developing new binders are potential strategies to bypass the complications arising from volumetric expansion, thereby maximizing the volumetric capacity of Si-based anode materials.
Self-Healing Materials

